Synergistic Interactions of Neighboring Platinum and Iron Atoms Enhance Reverse Water–Gas Shift Reaction Performance
- Type: Journal Article
- Author: Wang, Huilin; Bootharaju, Megalamane S.; Kim, Jeong Hyun; Wang, Ying; Wang, Ke; Zhao, Meng; Zhang, Rui; Xu, Jing; Hyeon, Taeghwan; Wang, Xiao; Song, Shuyan; Zhang, Hongjie
- Journal: Journal of the American Chemical Society
- Volume: 145
- Issue: 4
- Pages: 2264-2270
- Year: 2023
- DOI: 10.1021/jacs.2c10435
Abstract
The limitations of conventional strategies in finely controlling the composition and structure demand new promotional effects for upgrading the reverse water–gas shift (RWGS) catalysts for enhanced fuel production. We report the design and synthesis of a hetero-dual-site catalyst for boosting RWGS performance by controllably loading Fe atoms at the neighboring Pt atom on the surface of commercial CeO2. The Fe–Pt/CeO2 exhibits a remarkably high catalytic performance (TOFPt: 43,519 h–1) for CO2 to CO conversion with ∼100% CO selectivity at a relatively low temperature of 350 °C. Furthermore, the catalyst retains over 80% activity after 200 h of continuous operation. The experimental and computational investigations reveal a “two-way synergistic effect”, where Fe atoms can not only serve as promotors to alter the charge density of Pt atoms but also be activated by the excess active hydrogen species generated by Pt atoms, enhancing catalytic activity and stability.
Files and Links
- Url: https://doi.org/10.1021/jacs.2c10435
- Local Library: Zotero
Detail
Key points:
- Hetero-dual-site catalyst boosting RWGS reaction
- Controllably load Fe atoms at the neighboring Pt atom on the surface of CeO2
- High catalytic performance(TOFFe: 43519 h-1, selectivityCO: ~100%);high stability
- Fe atoms serve as promotors to alter the charge density of Pt atoms
- Fe atoms were activated by the excess active hydrogen species generated by Pt atoms
Learning points:
- Fe SAs is hardly observed by HAAD-STEM, but EDX mapping reveals them.
- XRD patterning was used to confirm the atomically dispersion.
- Mossbauer spectroscopy was performed to investigate the coordination environment of Fe species.
- Decreasing the total metal content leads to similar improvement (synergy still exists), which indicates that Pt an Fe atoms should not be randomly distributed.
- Hydrogen spillover can be detected by WO3.
Catalyst Factors Contributing to RWGS
Manufacturing of the catalysts with high metal dispersity, abundant oxygen vacancy, and an appropriate surface charge state.
Increasing the availability of active hydrogen species might be a potential route to overcome the performance limits.
- Single-atom dual sites were introduced into RWGS
Structure of Catalyst
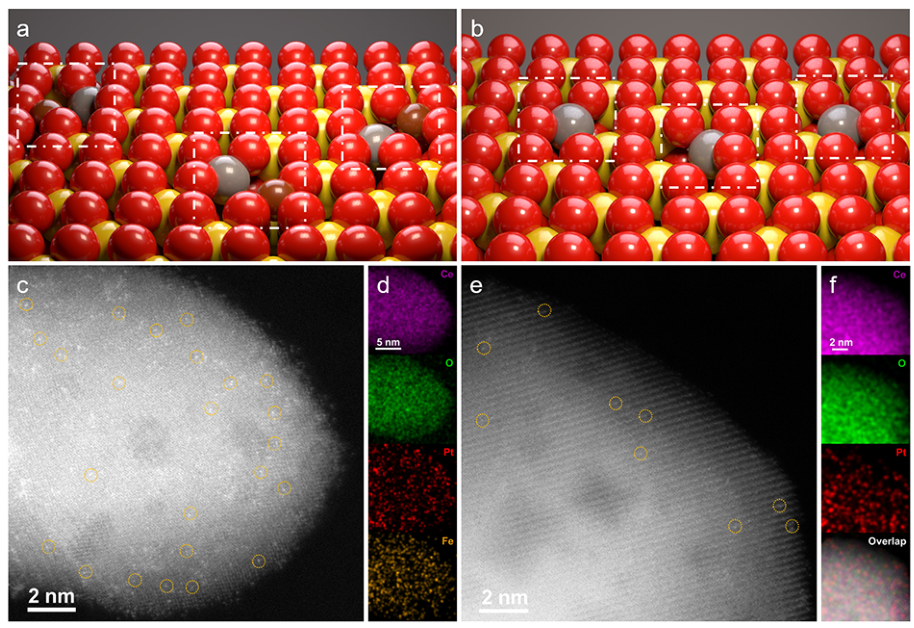
Pt species are atomically dispersed.
Both Pt and Fe species are uniformly distributed throughout the CeO2 support.
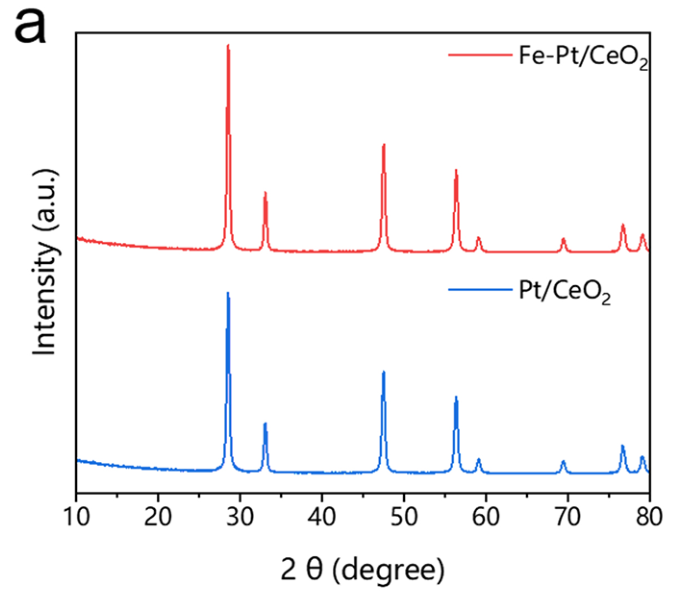
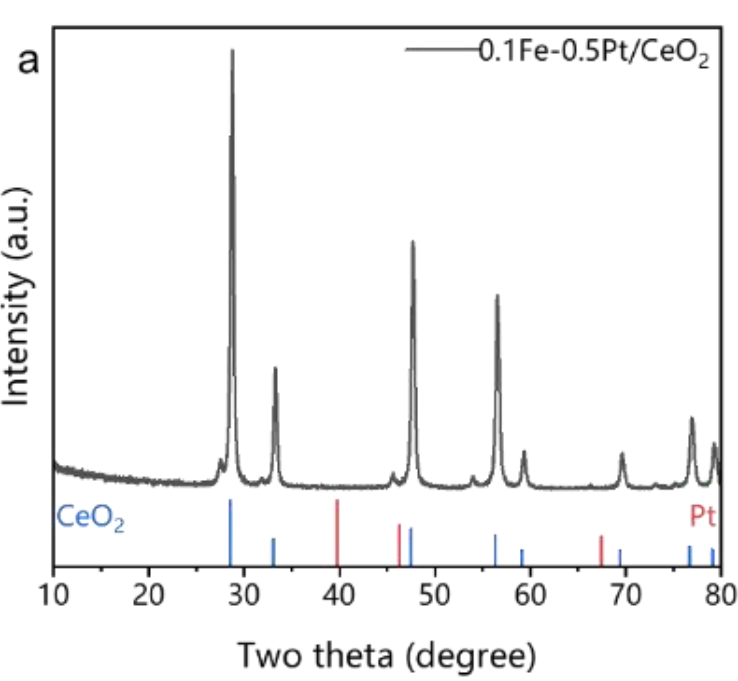
The absence of Pt diffraction peaks in the XRD pattern indicates its high atomic dispersion.
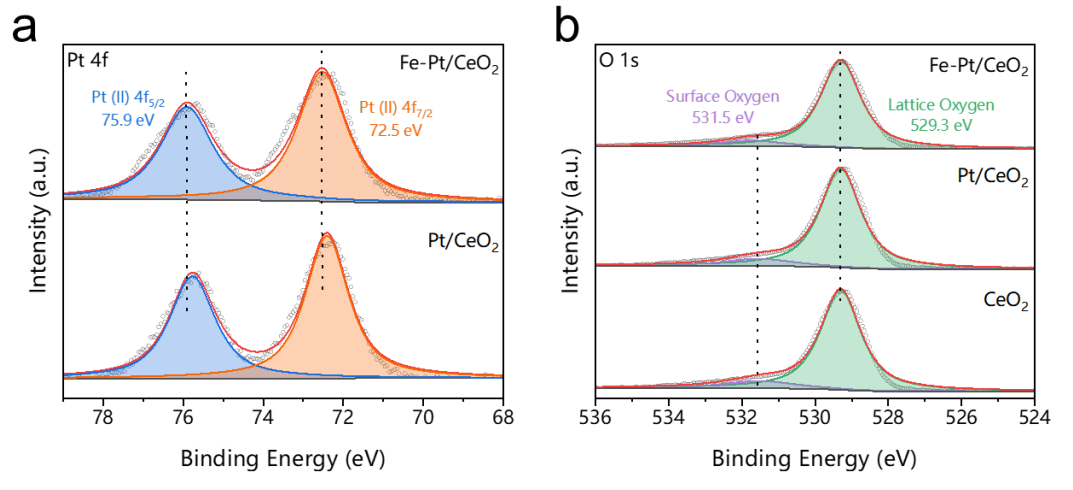
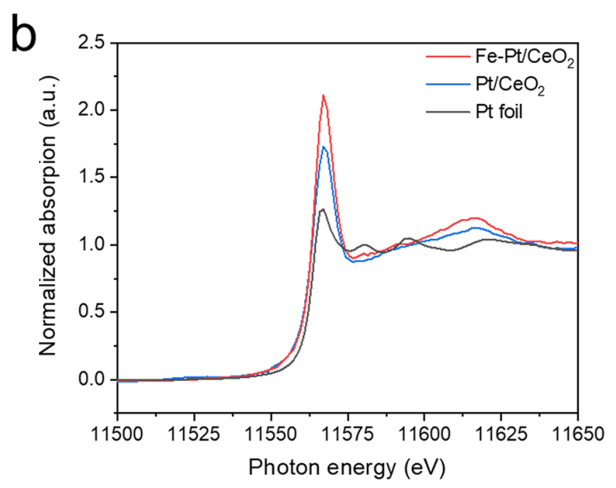 Pt species in Pt/Ce2 and Pt/Ce2 are in positively charged states.
Pt species in Pt/Ce2 and Pt/Ce2 are in positively charged states.
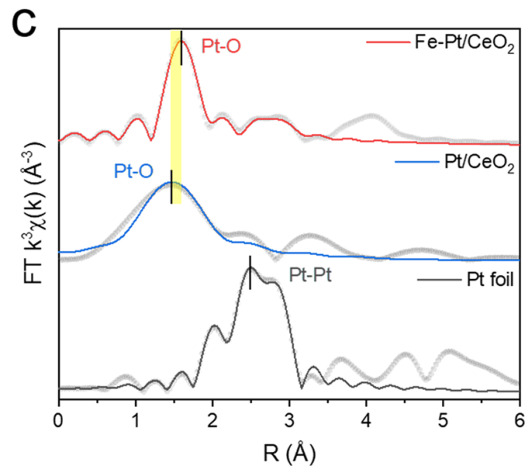
Pt−O bond in Fe−Pt/CeO2 is elongated due to nearby Fe atoms.
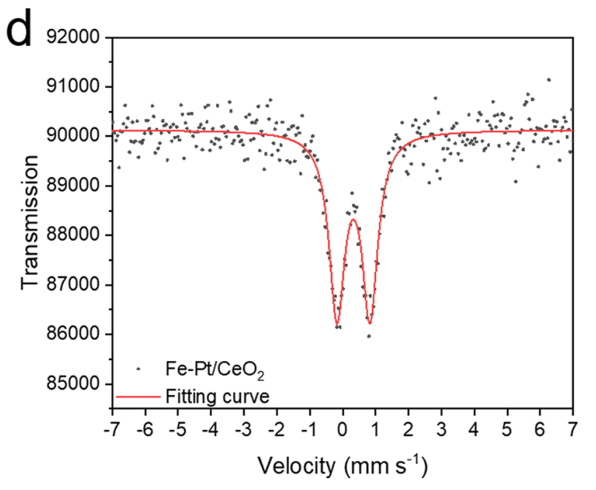 57Fe Mossbauer Spectroscopy: No metallic iron or iron oxide was identified, suggesting the atomic dispersion of Fe species.
57Fe Mossbauer Spectroscopy: No metallic iron or iron oxide was identified, suggesting the atomic dispersion of Fe species.
- Pt-O4-Fe model was constructed
 Electrons transfer from Pt to Fe.
Electrons transfer from Pt to Fe.
Catalytic Performance
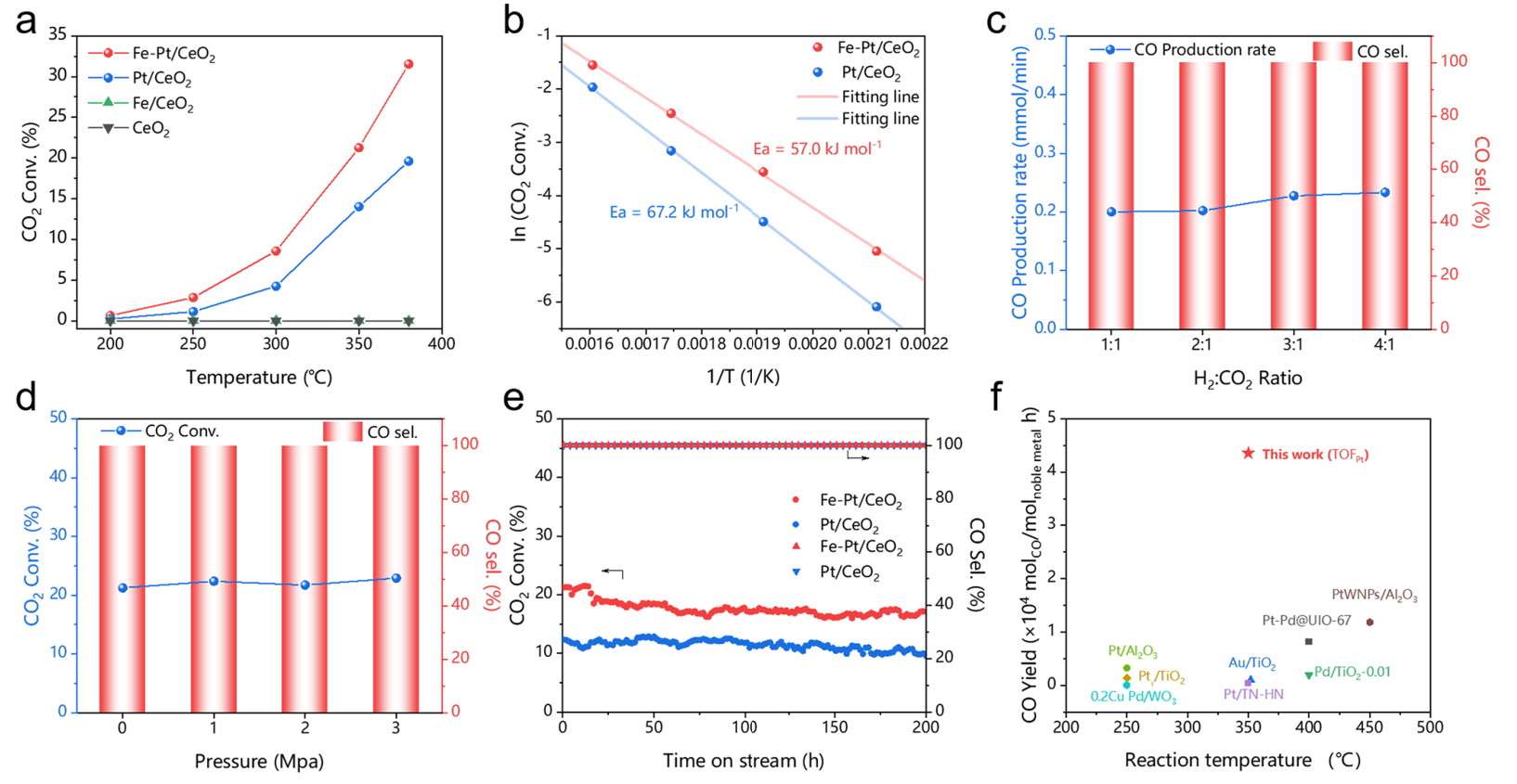 The stability is closely related to metal−support interactions but not from the Fe promoter.
The stability is closely related to metal−support interactions but not from the Fe promoter.
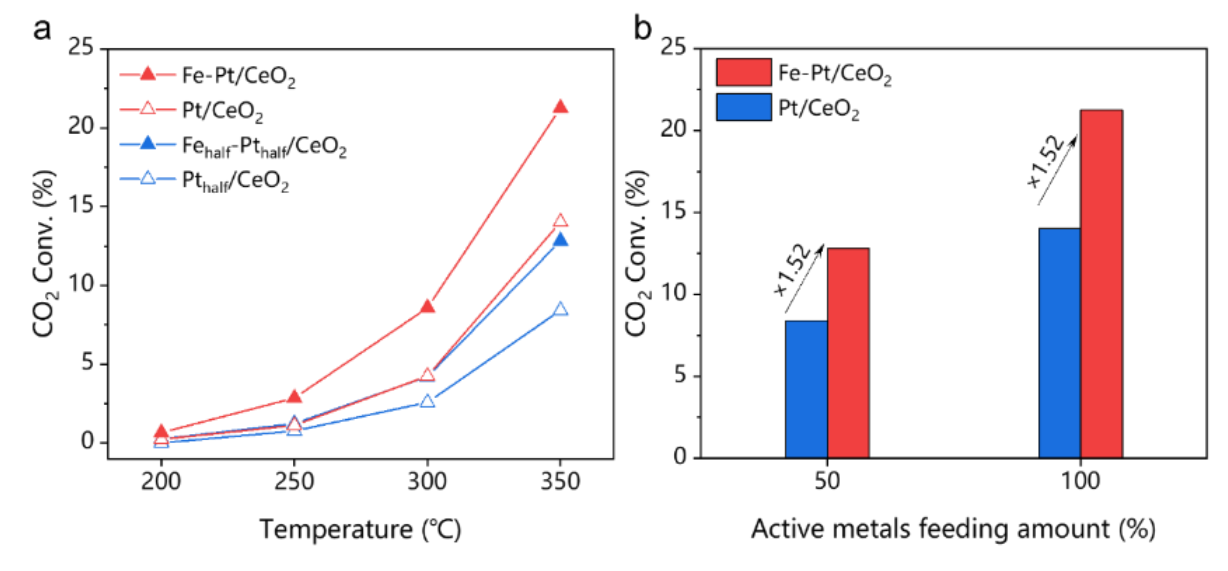
Decreasing the total metal content leads to similar improvement (synergy still exists), which indicates that Pt an Fe atoms should not be randomly distributed.
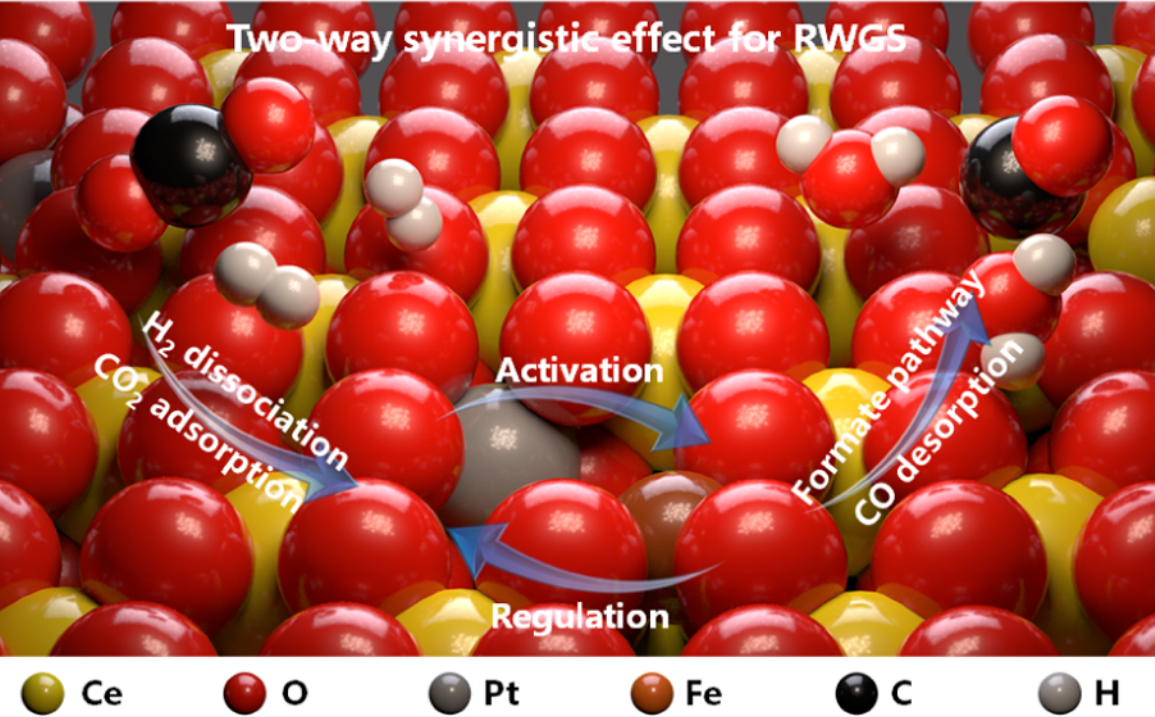
Two-way Synergistic Effect
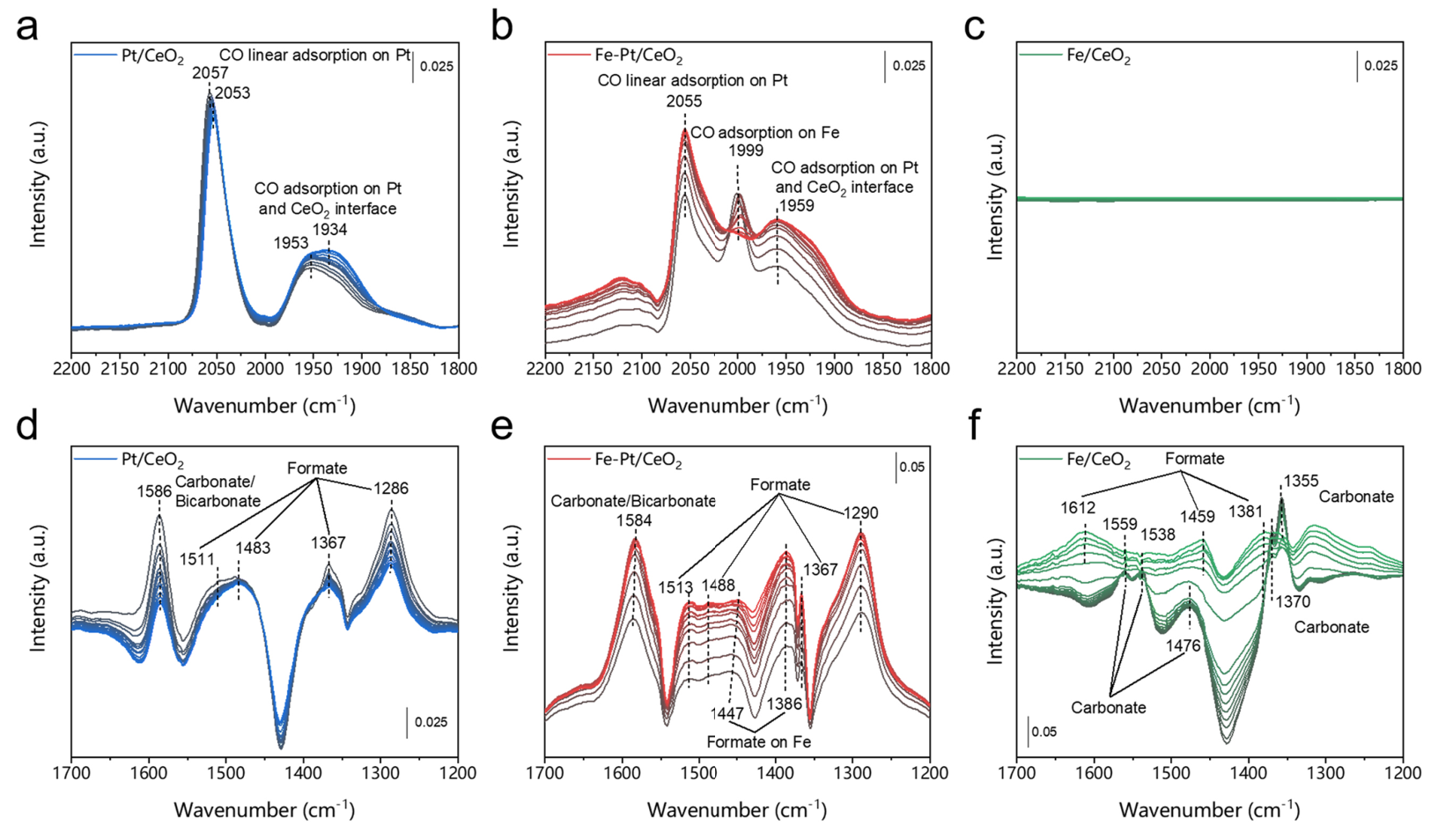
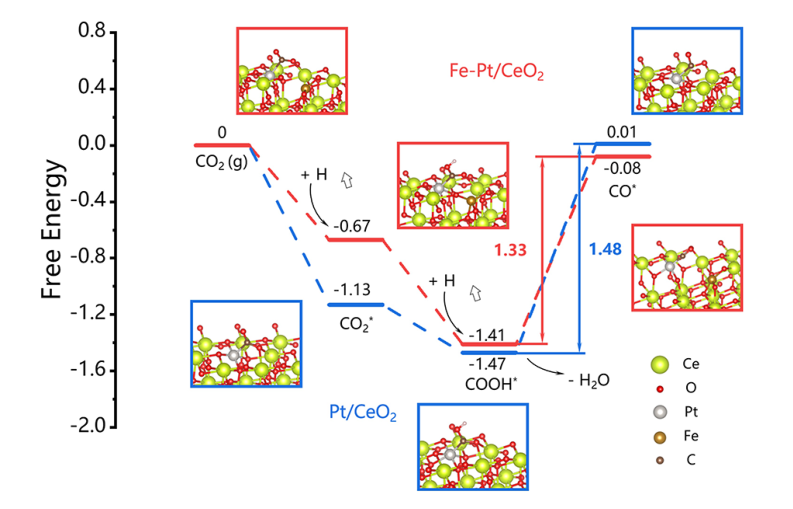
A possible reaction loop:
- In the first step, H2 molecules are dissociated on Pt sites to generate reactive hydrogen species.
- With the help of alkalinity of the CeO2 support, acidic CO2 molecules are readily adsorbed to the oxygen vacancies and can thus be activated.
- Next, Pt and Fe atoms individually serve as the active sites for formate intermediate generation.
- Subsequently, the intermediates are converted into CO* and desorbed from the catalysts in the form of CO molecules.